Abstract
Background: The biological and clinical impact of Cell-of-Origin (COO) subtypes based on gene expression profiling (GEP) of diffuse large B-cell lymphoma (DLBCL) is very limited in the Asian population, and the differences in the distribution and clinical impact of COO from North America and Europe are largely unknown.
Methods: We retrospectively analyzed 1,277 untreated de novo DLBCL patients, who were diagnosed and received rituximab-based immunochemotherapy from 2008 through 2018 in the Okayama Hematology Study Group (OHSG) from Japan, with available formalin-fixed paraffin-embedded (FFPE) tissues. We performed GEP-based COO classification, including double-hit signature (DHITsig), using the NanoString DLBCL90 assay. For comparison of distribution and clinical characteristics of COO subtypes between Asian and Western populations, an external real-world data set from BC Cancer was evaluated (J Clin Oncol. 2019;37(3):190-201). Immunohistochemistry staining and fluorescence in situ hybridization (FISH) were also performed to analyze phenotypic characteristics, such as MYC and BCL2 double hit lymphoma (DHL) using tissue microarray.
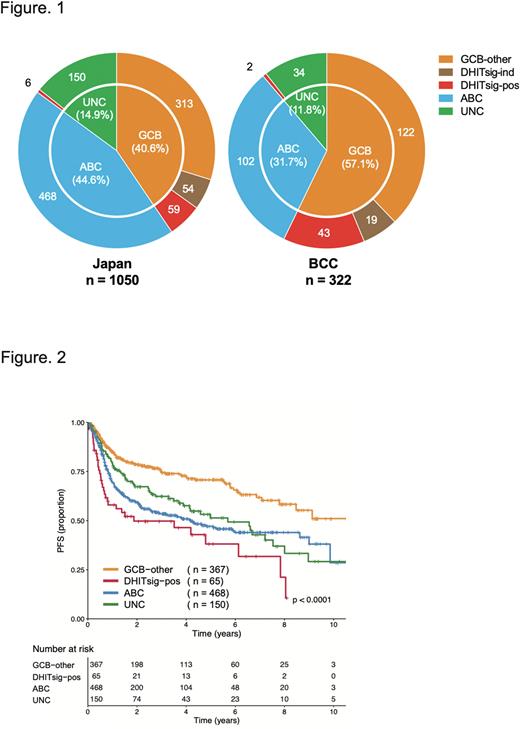
Results: The DLBCL90 assay was successfully performed on tumors from 1050 patients. The median age was 72 years (range 18 to 95), 591 patients (56.3%) were male, and 481 patients (45.8%) were international prognostic index (IPI) high or high-intermediate risk. The COO subtypes were assigned as follows: 432 patients (41.1%) with germinal center B-cell like (GCB)-DLBCL; 468 (44.6%) with activated B-cell like (ABC)-DLBCL; and 150 (14.3%) with Unclassified (UNC)-DLBCL, highlighting that the proportion of ABC-DLBCL in Japanese patients was significantly higher compared to the BCC cohort (ABC-DLBCL; 31.7% and GCB-DLBCL; 57.1%, P<0.001, Figure 1). Within GCB-DLBCL, 65 patients (15.0%) were assigned to the DHITsig-positive group. Compared to the DHITsig-negative and -indetermine GCB-DLBCL (GCB-other DLBCL) group, the ABC-DLBCL group was significantly older (median age; 70 vs 73, P<0.05) and showed elevated LDH and advanced stage (both P< 0.001). On the other hand, significant differences between DHITsig-positive DLBCL and GCB-other DLBCL was limited to the proportion of patients with elevated LDH (50.4% vs 73.8%, P<0.01). Within 198 cases with available FISH data, half of DHITsig-positive DLBCL cases were DHL (5/11 cases), consistent with the previous study. Of note, according to biopsy site, GCB-DLBCL cases were significantly enriched in the gastrointestinal tract (107/172 patients; 62%, P<0.001) and thyroid (14/16 patients; 87.5%, P<0.001), while ABC-DLBCL cases were enriched in the testicle (22/31 patients; 70.9%, P<0.01) and breast (14/19 patients; 73.6%, P<0.01).
With a median follow-up in living patients of 2.8 years, the estimated 5-year PFS of GCB-other DLBCL, DHITsig-positive DLBCL and ABC-DLBCL were 70.7%, 38.1%, 47.1%, respectively (P<0.0001, Figure 2). The estimated 5-year OS rates of those groups were 81.0%, 54.3%, 61.8%, respectively (P<0.0001), consistent with previous observations in the Western population. Within the DHITsig-positive DLBCL group, stratification factors of IPI had no prognostic impact.
Sixty-two cases in the cohort had available COO and DHITsig subtype assignments for both diagnostic and relapse biopsies, showing that 81.3% and 64.3% of ABC and GCB tumors at diagnosis remained the same subtypes at relapse, while only 27.3% (3/11 patients) of UNC-DLBCL were concordant. Compared to GCB-other, DHIT signature was found to be a strong poor prognostic factor for patients treated with salvage chemotherapies (hazard ratio 7.7, 95% confidence interval 1.5 to 39.1, P=0.014).
Conclusion: To our knowledge, this is the largest study of GEP-based COO determination including DHIT signature in the Asian DLBCL population. Our results indicate that new strategies are warranted for ABC-DLBCL and DHITsig-positive DLBCL patients due to poor outcome in the R-CHOP era.
Disclosures
Sunami:Chugai: Research Funding; GSK: Research Funding; Janssen: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Otsuka: Research Funding; Sanofi: Honoraria, Research Funding; Takeda: Research Funding; Celgene: Research Funding; BMS: Honoraria, Research Funding; AbbVie: Research Funding. Hiramatsu:Chugai pharmaceutical Co.: Honoraria; Nippon Shinyaku Co.: Honoraria; Bristol Myers Squibb: Honoraria; Sanofi K.K.: Honoraria. Yoshida:Kyowa Kirin: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Eisai: Honoraria; Jannsen: Honoraria; Nihonsinyaku: Honoraria; Ohtsuka: Honoraria; Symbio: Honoraria; Takeda: Honoraria; Dainihonsumitomo: Honoraria. Momose:Chugai Pharmaceutical Co.: Honoraria; Takeda Pharmaceutical Co.: Honoraria. Tamaru:Takeda Pharmaceutical Company Limited: Honoraria, Research Funding, Speakers Bureau; Chugai Pharmaceutical Co. LTD.: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Nichirei Bioscience Inc.: Research Funding; Ridgelinez Limited: Research Funding. Maeda:Eisai: Honoraria; Kyowa Kirin: Honoraria; Takeda: Honoraria; AstraZeneca: Research Funding; Astellas: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Novartis Pharma: Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Honoraria. Scott:Incyte: Consultancy; NanoString: Patents & Royalties; Janssen: Consultancy, Research Funding; Roche: Research Funding; AstraZeneca: Consultancy, Honoraria; Abbvie: Consultancy. Ennishi:Kyowa Kirin Pharmaceutical Co., Ltd.: Honoraria; Eisai Pharmaceutical Co., Ltd.,: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Nipponshinyaku Pharmaceutical Co., Ltd.,: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal